Packaging
Posts list
Drug Information Association (DIA) China
19 April 2023
From 2009 to 2023, for more than 14 years, DIA China has been dedicated to providing a neutral and transparent...
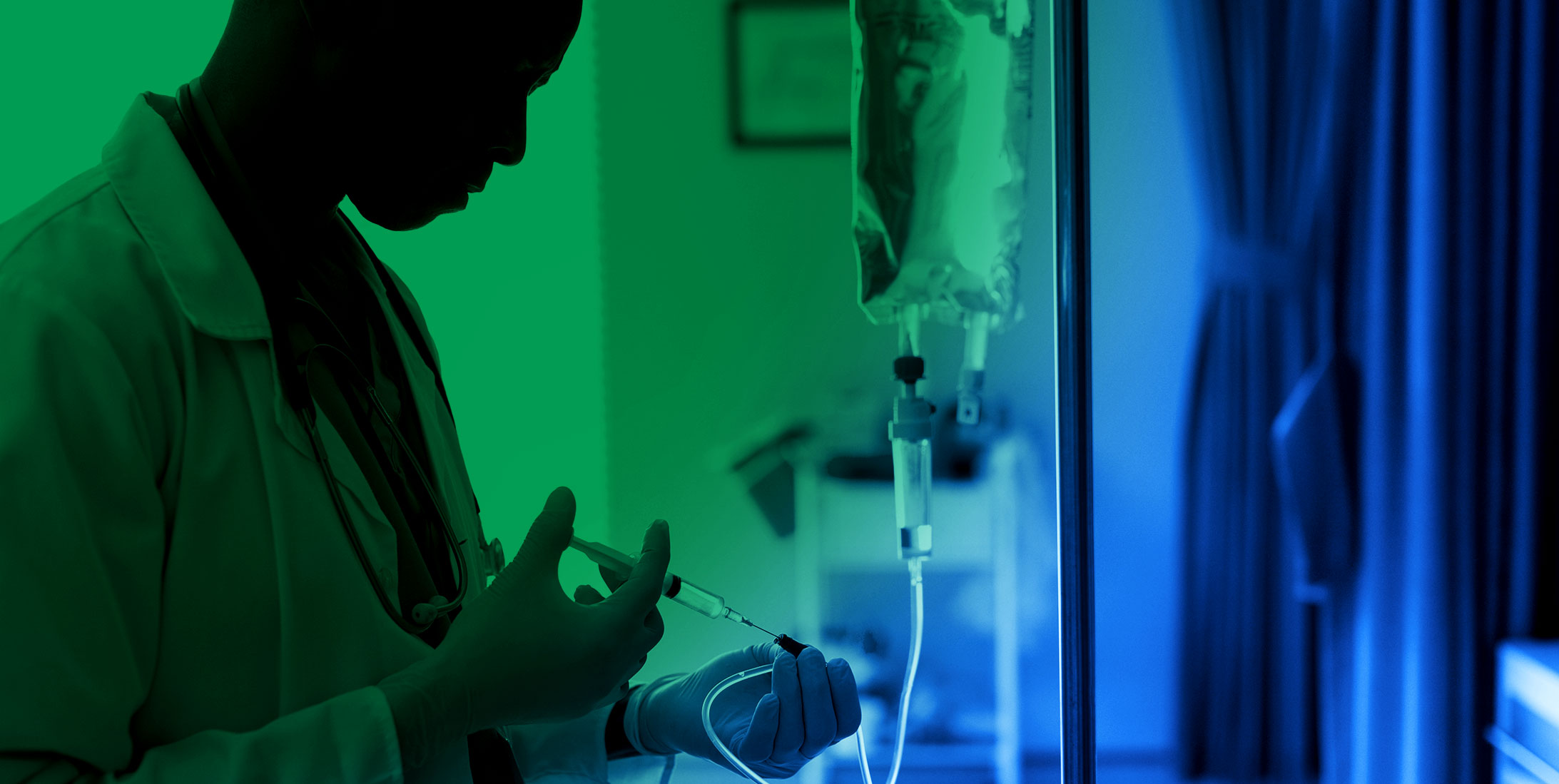
Marken Delivers Lifesaving Intravenous Therapy from Pennsylvania to Sites Across the USA Within 24 Hours
9 February 2023
Marken successfully delivered the time-critical intravenous therapy, Legalon, from its GMP Philadelphia depot to sites across the USA to treat...
New Import Control System 2 (ICS2) requirements for shipments entering or transiting the European Union
17 January 2023
Updated on 31 January, 2023 Author: Anna Cesar – Manager, Regulatory Compliance EMEA Effective March 1, 2023, Economic Operators will...
Happy Holidays from Marken
21 December 2022
At Marken, we are passionate about patients – always finding a way to deliver for our clients – on-time, within...
Marken Successfully Ships Lifesaving Treatments For Humanitarian Aid In Ukraine
24 June 2022
Marken successfully transported critical compassionate use medicinal products and supplies as part of a humanitarian effort in Ukraine. Mitigating the...
CDC Noninfectious Certification Statement
20 April 2022
Recently the Centers for Disease Control (CDC) reminded the trade community that shipments of noninfectious diagnostic specimens must be accompanied...
The New European Medical Device (MDR) and In-Vitro Regulation (IVDR)
25 May 2021
Author: Anna Cesar | Associate, Regulatory Compliance The transition period for the new European Medical Device (MDR) and In-Vitro Regulation...
Marken Customizes Supply Chain Solutions for Regional Clinical Vaccination Program
9 March 2021
Marken Customizes Supply Chain Solutions for Regional Clinical Vaccination Program A regional Ministry of Health required its limited supplies of...
Implementing Resilience in the Clinical Trial Supply Chain
11 November 2020
AUTHOR: Nina Vas | Vice President, Clinical Distribution, Cell and Gene Supply Chain Since January 2020, the clinical trials ecosystem...
New Marken Overpacks immediately Identify Time Critical Shipments
30 July 2020
GREEN COLOR WILL INCREASE VISIBILITY Research Triangle Park, N.C., July 30, 2020 – Marken today announced they have transitioned their...
Marken Launches COVID-19 Test Kit Production Unit
9 June 2020
Miami Branch will Grow to Accommodate the Increased Demand Research Triangle Park, NC, June 9, 2020 – Marken today announced...
Marken Continues to Deliver Critical Medications
20 March 2020
Rescues cross-border shipments for two Canadian pediatric patients About Marken Marken is the clinical precision logistics and advanced therapy subsidiary...
Understanding why we must adapt, continuously asking ourselves how we can change what matters is how we keep on delivering it.
Login and quick links